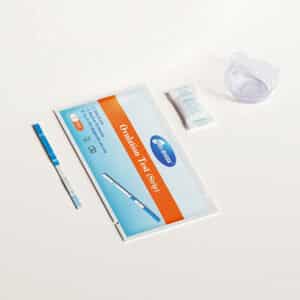
Status COVID-19/Flu A&B POC Test Kit (Pack of 25 tests)
$499.75/box of 25 tests ($19.99/test)
QUICK SHIPPING! Ground, 2-day, and overnight (M-F) shipping available on in-stock test kits! All in-stock parcel shipment orders received before 1pm EST/10am PST ship same-day!
*CLIA Number Required
Fast & Secure Checkout
Description
- A rapid immunoassay for the simultaneous direct detection and differential diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens
- Anterior nasal swab specimen
- Flu A – Sensitivity 91.4%, Specificity 95.7%
- Flu B – Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
- Fact sheet for Healthcare providers HERE
- Fact sheet for Patients HERE
- CLIA Number Required
Contents Include:
- Status COVID-19/Flu A&B test devices (25): The test strip in each device contains mouse monoclonal antibodies to nucleocapsid protein of influenza A, influenza B and SARS-CoV-2. The device is individually pouched.
- Extraction Reagent in capsules (25): For use with swab specimens; 300 µL of Phosphate buffer with detergents and preservative
- Sterile Swabs (25): For swab specimen collection
- Positive Control Swab (1): Influenza A, B, and SARS-CoV-2 antigen (non-infective recombinant nucleocapsid protein)
- Negative Control Swab (1): Inactivated Group B Streptococcus antigen (non-infective)
- Package Insert /Instructions for use (1)
- Quick Reference Instructions (1)
- Materials Required, But Not Provided
- Timer
Using Status COVID-19/Flu A&B POC Tests
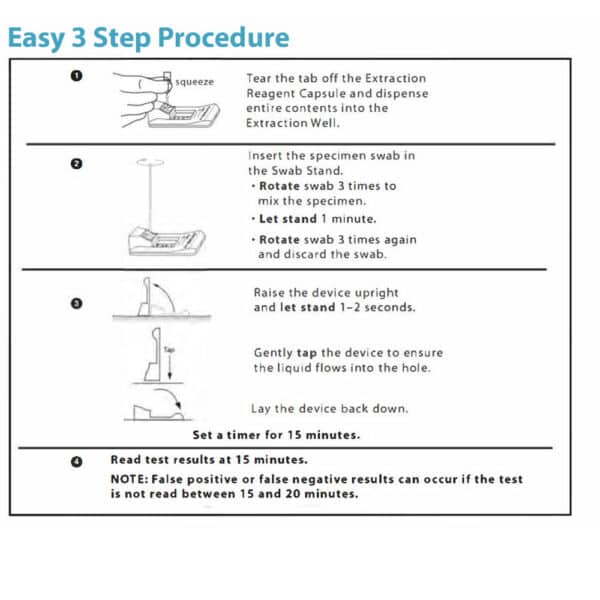
- Tear the tab off the extraction reagent capsule and dispense entire contents into the extraction well.
- Nasopharyngeal Swab Specimen Collection:
- Gently and slowly insert a minitip swab with a flexible shaft through the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril, indicating contact with the nasopharynx.
- Leave swab in place for several seconds to absorb secretions.
- Slowly remove swab while rotation.
- Anterior Nasal Swab Specimen Collection:
- Use a flocked swab provided in the kit and insert the entire soft end of the swab into the nostril no more than 3/4 of an inch (1.5cm) into the nose.
- Slowly rotate the swab, gently pressing against the inside of the nostril at least 4 times for a total of 15 seconds.
- Get as much secretion as possible on on the soft end of the swab. Gently remove the swab.
- Nasopharyngeal Swab Specimen Collection:
- Insert the specimen swab in the swab stand.
- Rotate swab 3 times to mix the specimen
- Let stand 1 minute
- Rotate swab 3 times again and discard the swab
- Raise the device upright and let stand 1-2 seconds.
- Gently tap the device to ensure the liquid flows into the hole.
- Lay the device back down
- Set a timer for 15 minutes
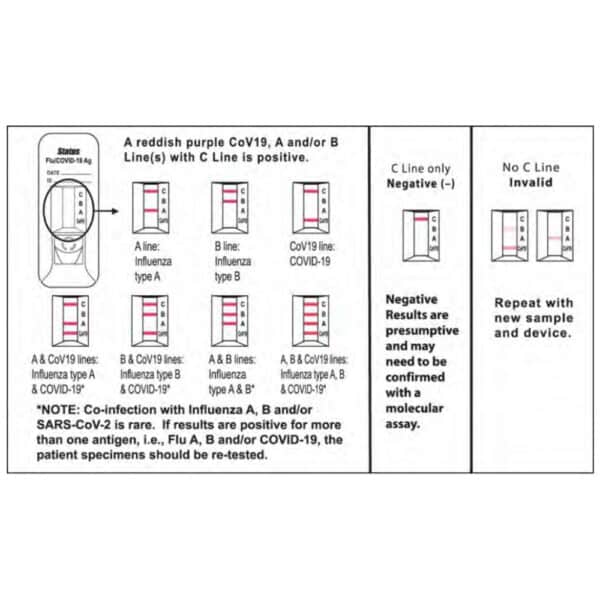
- Read test results at 15 minutes.
NOTE: False positive or false negative results can occur if the test is not read between 15 and 20 minutes.
Status COVID-19/Flu A&B POC Tests for Sale Online
We source the highest quality PPE to protect against and prevent the spread of illness. We only work with respected vendors and carefully vet all products. With 24/7 ordering and exceptional customer service and bulk ordering, SUNLINE Supply makes it easy to get the test kits you need. Check out our complete line of personal protective equipment online today and get the supplies you need!
Additional information
| Weight | .71 lbs |
|---|---|
| Dimensions | 5 × 9.35 × 2.66 in |
- Printed Expiration Date: March 31, 2024
- Extended Expiration Date: December 31, 2024
- Expiration Date: If you receive a box that has an expiration date that has passed, please do not be concerned. The state department has granted an extended expiration life FOR THIS PRODUCT. Please see extension letter HERE.
EUA Info from the FDA:
- This test is not yet approved or cleared by the United States FDA. When there are no FDA-approved or cleared tests available, and other criteria are met, FDA can make tests available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA for this test is supported by the Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the emergency use of in vitro diagnostics for the detection and/or diagnosis of the virus that causes COVID-19. This EUA will remain in effect (meaning this test can be used) for the duration of the COVID-19 declaration justifying emergency of IVDs, unless it is terminated or revoked by FDA (after which the test may no longer be used)