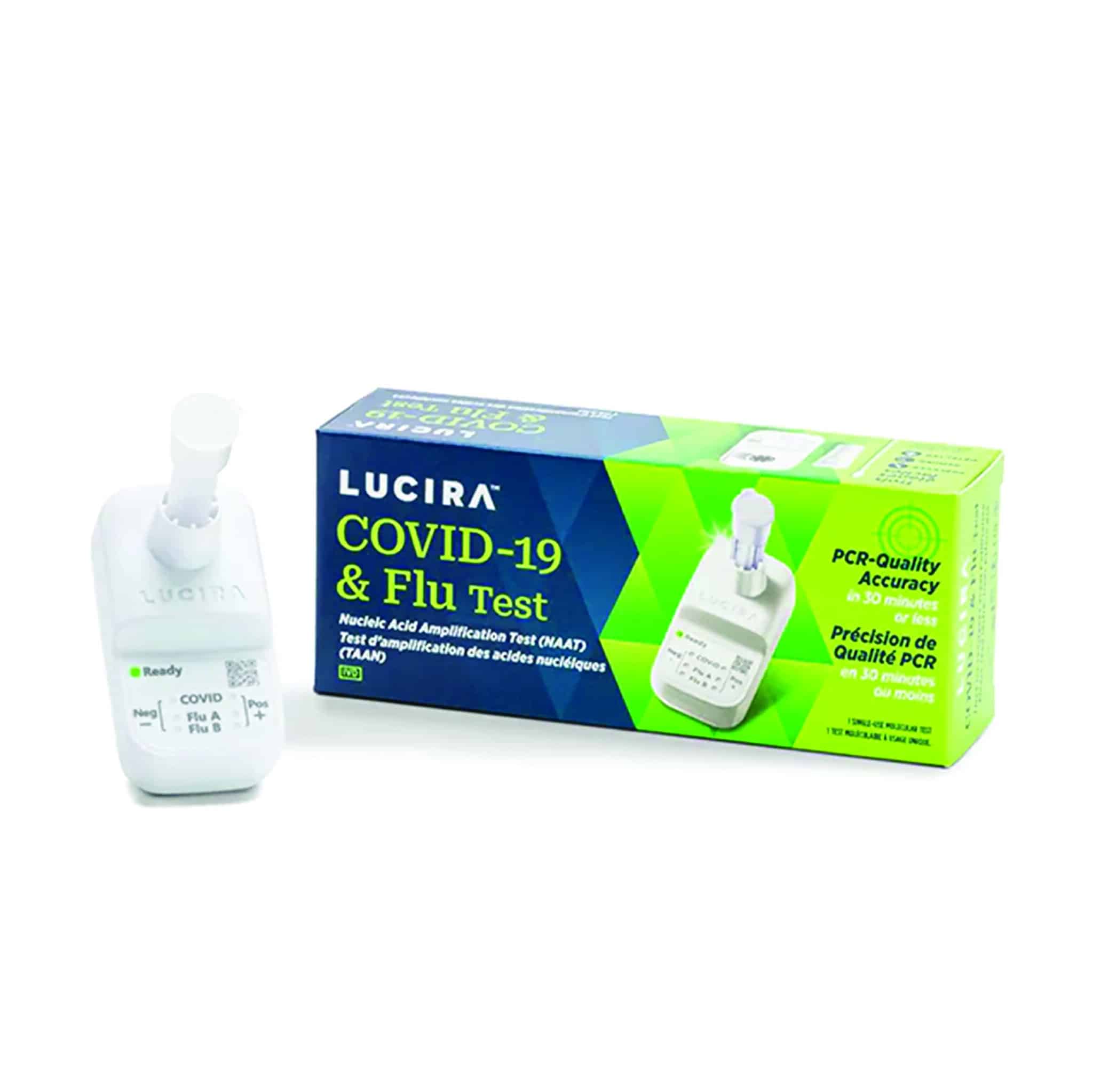
Lucira – COVID/Flu At-Home Rapid PCR Test Kit
Contact us to Purchase
For use under an Emergency Use Authorization (EUA) only for the duration of the COVID-19 declaration justifying the emergency use of in vitro diagnostics (IVD), unless it is terminated or revoked by the FDA (after which the test may no longer be used)
QUICK SHIPPING! Ground, 2-day, and overnight (M-F) shipping available on in-stock test kits! All in-stock parcel shipment orders received before 1pm EST/10am PST ship same-day!
Contact Us to Purchase Fast & Secure Checkout
Description
- The Lucira COVID/Flu Test Kit uses molecular amplification technology to simultaneously and rapidly identify and distinguish SARS-CoV-2, Influenza A, and Influenza B viral RNA. This method targets SARS-CoV-2 RNA for early infection detection, in contrast to protein-focused antigen tests.
- The Lucira COVID/Flu All-In-One Test meets requirements for testing when traveling between the US and to Canada, as well as many other international locations. Includes access to results documentation for verification.
- Lucira offers a free LUCI PASS(TM) digital verified text result back to a users phone:
- Accessed via text and does not require downloading an app
- Opt-in feature for public health reporting for users who wish to transmit their results to the relevant public health authorities
- Molecular test, not an Antigen test: Just like lab-based PCR tests, this test amplifies virus genetic material for accurate, early detection.
- Can detect all COVID-19 variants of concern, including the Omicron variant and subvariant BA.2, as well as Influenza A and B.
- Results in as little as 11 minutes for positive results, and 30 minutes for negative results.
- Instructions for Use HERE
- Video Instructions for Use at Home HERE
- Fact Sheet for Healthcare Provider HERE
- Read about the difference between Antigen tests and Antibody tests
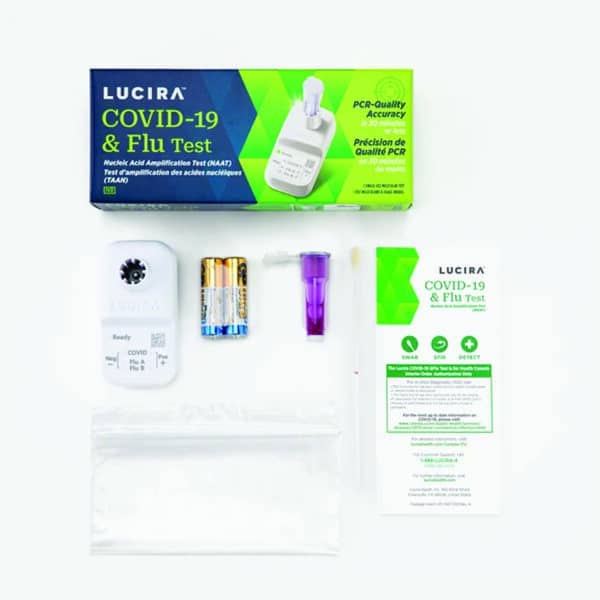
Contents Include:
- Lucira test unit
- Sample vial
- Nasal swab: swab for anterior nasal specimen collection
- 2 AA batteries
- Plastic disposable bag
- Instructions
Lucira™ COVID/Flu Home Tests are intended for use at home, without the need for a medical professional. Any adult can use these tests to gather samples and test for COVID/Flu at home.
How To Use Lucira™ OTC COVID/Flu PCR Testing Kits
Using the test is simple, and each kit comes with everything you need to complete the process. Detailed and illustrated instructions are also included.
Follow these steps to administer this COVID/Flu test:
- Read the directions and examine the kit components.
- Disinfect the flat surface where you will be conducting the test and carefully wash your hands.
- Open battery door and insert batteries. Check that ready light is on.
- Open sample vial pouch 2.
- Remove sample vial seal then gently set in test unit but do NOT push the vial down.
- Remove swab and hold with handled end. Do not set swab down.
- Tilt head back and gently insert swab tip until it is fully inside your/patient nostril and you meet resistance.
- Once swab tip is fully inside nostril, roll the swab 5 times around the inside walls of your nostril. The swab should be touching the walls of the nostril as you rotate.
- Repeat swab step in other nostril.
- Insert swab into the sample vial until it touches the bottom and mix sample by stirring around the sample vial 15 times. Discard swab.
- Snap cap closed and press vial down into test unit until it clicks.
- Ready light will start blinking when test is running. If ready light is not blinking within 5 seconds, use palm of your hand to press down more firmly to start test. Do not move test unit once the test has started running.
- Wait 30 minutes. Done light will display when test is ready in 30 minutes.
- Next, please use Luciras secure LUCI portal to receive a verified test result on your phone and transmit your result to public health agencies. To get started, please text the word ‘LUCI’ to 44544.
What is LUCI PASS™ and How Do I Use IT?
How do I share my LUCI results?
Your LUCI PASS is stored in your LUCI account for 7 days and is accessed by texting “RESULTS” to 44544 from a US phone number or by logging into lucipass.com.
You can also download and email a PDF version of your LUCI PASS. Once downloaded, you can print the PDF.
How long is my LUCI PASS good for?
Your LUCI PASS lasts for 7 days and then will be cleared from your account. Check the requirements for where you plan to use your LUCI PASS, as many partners require test results within 48 or 72 hours (2-3 days).
What does LUCI PASS display?
Your LUCI PASS will display:
Your name, date of birth and optional selfie; Your verified test result, including the test results date and time; Test accuracy, clinical performance and ability to detect COVID-19 variants; A link to Lucira’s FDA Emergency Use Authorization (EUA)
How does LUCI PASS work for travel?
Always check with your airline for details and restrictions on using your Lucira test results for travel to your destination. Restrictions vary between destinations.
When an airline requests proof of a negative COVID-19 test result, it’s usually best to download your LUCI PASS as a PDF and upload the PDF to the airline website. Once downloaded, you can print a hard copy of the PDF and bring it with you, in addition to displaying LUCI PASS on your smartphone at the airport.
Note that for travel to Hawaii, Lucira is now available through AZOVA’s Hawaii Travel Program.
How do I share my Lucira test results with my airline?
Always check with your airline for details on sharing your test results. Each airline has different requirements and we recommend downloading your LUCI PASS as a PDF file and then uploading that PDF file to the airline’s website.
You can also print a hard copy of the downloaded PDF and bring it with you to the airport, in addition to displaying LUCI PASS on your smartphone at the airport.
Can I use LUCI with the Lucira COVID-19 All-In-One (prescription) Test Kit?
Yes, you can obtain a LUCI PASS if your healthcare provider prescribes you the Lucira COVID-19 All-In-One Test. You will need the test kit box and your resulted device. Public health reporting is still the healthcare provider’s responsibility even if the patient uses LUCI.
Will my personal or health information be shared?
LUCI can optionally transmit your test result to your state’s public health authority. This is optional. Your test results are deleted from the LUCI system after 7 days. Lucira will not share your personal or health information without your express consent.
Lucira™ COVID/Flu PCR Home Tests Provide Accurate Results
Depending on your location and community’s access to COVID/Flu testing, home tests may be the best way for you to verify whether your employees are positive. Through comparisons between Lucira™ COVID/Flu test kit results and laboratory PCR tests, we know that the Lucira™ Check It test kit is up to 98% effective. Get a reliable result when you need to confirm that your team is ill or feel confident working and going out into your community knowing your employees are in good health.
Buy Lucira™ COVID/Flu PCR Tests at SUNLINE Supply
When you need Lucira™ COVID/Flu at-home test kits, shop with SUNLINE Supply for 24/7 sales availability. Our antigen test kits for sale are ready for same-day shipping if you order before noon EST. Buy your Lucira™ COVID/Flu test kits from a trusted company like SUNLINE Supply today!
Additional information
| Weight | 0.14 lbs |
|---|---|
| Dimensions | 5.50 × 6.00 × 1.50 in |
Expiration Dates:
- Printed Expiration Date: July 11, 2023
- Extended Expiration Date: January 7, 2024
- Expiration Date: If you receive a box that has an expiration date that has passed, please do not be concerned. The state department has granted an extended expiration life FOR THIS PRODUCT. The shelf life for the Lucira COVID-19 & Flu Test Kit has been extended to 18 months. For COVID-19 tests, manufacturers are required to label products based on real-time stability studies. Lucira Health completed the real-time stability testing to support an 18-month shelf life.
EUA Info from the FDA:
- The Lucira COVID/Flu All-In-One Rapid PCR Test Kit is a single-use test kit intended to rapidly identify and distinguish SARS-CoV-2, Influenza A, and Influenza B viral RNA. This method targets SARS-CoV-2 RNA for early infection detection, in contrast to protein-focused antigen tests.. This test is authorized for prescription home use with self-collected nasal swab samples in individuals aged 14 and older who are suspected of COVID/Flu by their healthcare provider.